Nội dung chính
- 1 What is the product of HCl and MnO2
- 2 What type of reaction is HCl + MnO2
- 3 How to balance HCl + MnO2
- 4 Is HCl + MnO2 titration
- 5 HCl + MnO2 net ionic equation
- 6 HCl + MnO2 conjugate pairs
- 7 HCl + MnO2 intermolecular forces
- 8 HCl + MnO2 reaction enthalpy
- 9 Is HCl + MnO2 a buffer solution
- 10 Is HCl + MnO2 a complete reaction
- 11 Is HCl + MnO2 an exothermic or endothermic reaction
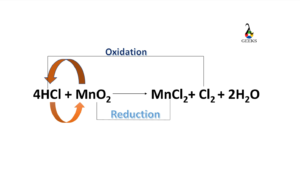
- 12 Is HCl + MnO2 a redox reaction
- 13 Is HCl + MnO2 a precipitation reaction
- 14 Is HCl + MnO2 reversible or irreversible reaction
- 15 Is HCl + MnO2 displacement reaction
Chúng tôi rất vui được chia sẻ kiến thức sâu sắc về từ khóa 15 Facts on HCl + MnO2: What, How To Balance & FAQs. Bài viết mno2 hcl tập trung giải thích ý nghĩa, vai trò và ứng dụng của từ khóa này trong tối ưu hóa nội dung web và chiến dịch tiếp thị. Chúng tôi cung cấp phương pháp tìm kiếm, phân tích từ khóa, kèm theo chiến lược và công cụ hữu ích. Hy vọng thông tin này sẽ giúp bạn xây dựng chiến lược thành công và thu hút người dùng.
Hydrochloric acid is a colorless solution with a distinctively pungent smell whereas MnO2 has an asphalt odor. Let us read how HCl and MnO2 react with each other.
Bạn Đang Xem: 15 Facts on HCl + MnO2: What, How To Balance & FAQs
Manganese dioxide is a blackish or brownish, inorganic compound, naturally occurring compound. It is used as a pigment and reagent in organic synthesis. MnO2 has a molar mass of 86.9268 g/cm3. HCl is a very strong acid.
In detail, this article will inspect key features of the HCl and MnO2 reaction, like products, net ionic equation, type of reaction, and intermolecular forces of interactions.
What is the product of HCl and MnO2
Scacchite (MnCl2), dichlorine (Cl2), and water (H2O) are products of the reaction HCl + MnO2.
HCl +MnO2 = MnCl2 + Cl2 + H2O
What type of reaction is HCl + MnO2
The reaction type of HCl +MnO2 is a redox reaction.
How to balance HCl + MnO2
The overall balanced molecular equation for HCl +MnO2 is –
Xem Thêm : Đội bóng Thái chính thức giải mã các bí ẩn về vụ bận bịu kẹt
4HCl +MnO2 = MnCl2+ Cl2+ 2H2O
The balanced equation is derived by using the following method:
- All the compounds of the reaction are labeled with a different variable.
- a MnO2 + b HCl = c MnCl2+ d Cl2 + e H2O
- Create a system of equations for each reactant and product side element.
- Mn = 1a+1c, O=2a+1e, H= 1b+2e, Cl = 1b=2c=2d
- By using the Gaussian elimination or substitution method, solve all equations for each variable.
- a= 1(MnO2), b=2(HCl), c=1(MnCl2), d=1(Cl2), f=2(H2O)
- Substitute for each of the coefficients.
- Thus, the balanced equation is as follows:
- 4HCl + MnO2 = MnCl2+ Cl2 + 2H2O
Is HCl + MnO2 titration
Titration between HCl and MnO2 is not feasible because of HCl, as due to its oxidizing property Cl2 is liberated, which does not turn the medium into acidic. Whereas MnO2 is a water-insoluble solid, which makes stirred titration mixture look like mud.
HCl + MnO2 net ionic equation
The net ionic equation for HCl + MnO2 is as follows;
2HCl + MnO2 + 2H+ = Mn2+ + Cl2 + 2H2O
Following are steps to find the net ionic equation for the reaction.
- The given equation is –
- HCl(aq) + MnO2 (s)= Mn2+(aq) + Cl2(aq)
- Mn oxidation number is decreased from +4 to +2. This implies that Mn is reduced.
- In Cl, it is oxidized because its oxidation number is increased from -1 to 0.
- Following are oxidation and reduction half-reactions of the given reaction-
- HCl =Cl2 , MnO2 =Mn2+
- Now, balance each element except H and O in the equation, and the equations become-
- 2HCl = Cl2 , MnO2 =Mn2+
- Now balance oxygen and hydrogen using water and proton on both sides, so the we get following equations –
- 2HCl = Cl2 + 2H+
- MnO2 + 4H+ = Mn2+ + 2H2O
- By balancing charges on both sides we get equations as,
- 2HCl =Cl2 + 2H+ + 2e- (i)
- MnO2 + 4H+ + 2e- = Mn2+ + Cl2 + H2O (ii)
- Now Địa chỉ cửa hàng both the equations (i) and (ii), we get final net ionic equation as –
- MnO2 + 2HCl +2H+ = Mn2+ + Cl2 + 2 H2O
HCl + MnO2 conjugate pairs
The conjugate acid or base pairs of HCl + MnO2 are-
- HCl (Conjugate base) = Cl –
- HCl (Conjuagte acid) = H3O+
- MnO does not have conjugate pairs as it is an amphoteric oxide.
HCl + MnO2 intermolecular forces
The different types of intermolecular forces or interaction involved in HCl + MnO2 reaction are-
- In HCl, there are two types of intermolecular forces present, dipole-dipole interactions and London dispersion forces. Among these dipole-dipole interactions, forces are stronger.
- In MnO2, strong ionic forces are present with high electrostatic forces.
- Water (H2O) forms H- bonding.
HCl + MnO2 reaction enthalpy
The reaction enthalpy of HCl + MnO2 is +272.3 KJ/mol.
- The total enthalpy of reaction = total of product enthalpy – total enthalpy of reactant
- Thus for the reaction- 4HCl + MnO2 = MnCl2 + Cl2 + 2H2O
- (-369.3) + (- 520)= (- 481.2) + (- 136)
- (-889.3) = (- 617)
- Reaction enthalpy = (-617) – (-889.3)
- The total enthalpy of reaction= 272.3 KJ/mol
Is HCl + MnO2 a buffer solution
Xem Thêm : Những câu giới thiệu bản thân hài hước trên Facebook – Tin Đẹp
HCl + MnO2 when mixed does not form a buffer solution because of HCl; as it is a very strong acid, and generally buffer solutions are prepared from a weak acid and its respective conjugate base.
Is HCl + MnO2 a complete reaction
The reaction between HCl + MnO2 is a complete reaction, and the products (MnCl2, Cl2, and H2O) so formed are not converted back to reactants, and further, there are no products formed.
Is HCl + MnO2 an exothermic or endothermic reaction
The HCl + MnO2 reaction is an endothermic reaction, as the total enthalpy of the reaction is positive (272.3 KJ/mol).
Is HCl + MnO2 a redox reaction
HCl + MnO2 is a redox reaction as Mn4+ gets reduced to Mn2+, and 2Cl- gets oxidized to 2Cl, where MnO2 acts as an oxidizing agent, and HCl acts as a reducing agent.
Is HCl + MnO2 a precipitation reaction
The reaction HCl + MnO2 is an example of a precipitation reaction, as a brownish precipitate of MnCl2 is formed at the end of the reaction.
Is HCl + MnO2 reversible or irreversible reaction
HCl + MnO2 is an irreversible reaction because chlorine gas (Cl2) is evolved at the end of the reaction and thus the reaction cannot be reversed back.
Is HCl + MnO2 displacement reaction
The HCl and MnO2 reaction is a type of double-displacement reaction, because Mn displaces Cl from HCl, and O is displaced to H from MnO2.
Conclusion
At last, when the reaction takes place between HCl and MnO2 the products formed are MnCl2, H2O, and Cl2. During this production of chlorine gas takes place at the end of the reaction. Manganese dioxide (MnO2 ) is mainly used to manufacture dry-cell batteries.
Nguồn: https://kengencyclopedia.org
Danh mục: Hỏi Đáp





